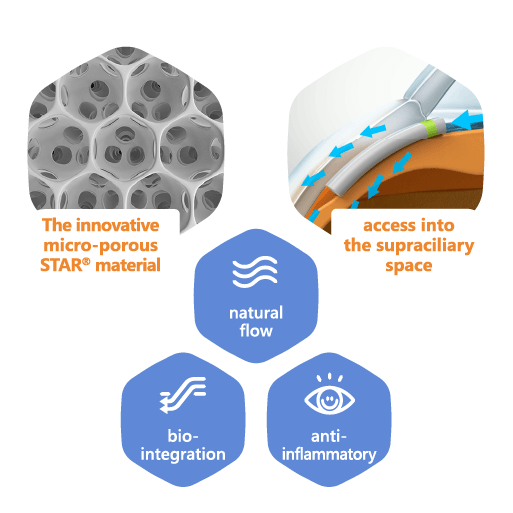
Delivering breakthrough eye care solutions
MINIject® is seeking to transform the glaucoma treatment landscape with its distinctive, tissue-integrating STAR® material. MINIject® is currently the only commercially available minimally invasive glaucoma surgery (MIGS) implant that enhances natural flow in the supraciliary space, and has been shown to deliver safe, meaningful and sustained control of intraocular pressure (IOP).